


The number of nucleons present in an atom is called mass number. Protons and neutrons reside in the nucleus of an atom, and they, in together, are called nucleons. ISBN 978-0-19-539131-2.An atom consists of electrons, protons, and neutrons. “The Origins of the Symbols A and Z for Atomic Weight and Number”. Blackwell Scientific Publications: Oxford. Compendium of Chemical Terminology (2nd ed.) (the “Gold Book”). It is typically located as a subscript to the left of the element symbol. The atomic number is the smaller of the two numbers in the symbol. For example, if the symbol is 14 6C, the number “6” is listed. Usually, both the mass number and atomic number are given in an isotope symbol.Look for the symbol “C” on the periodic table to get the atomic number. For example, if the symbol is 14C, you know the element symbol is C. Find the atomic number from the isotope symbol the same way.(The exception is Mendeleev’s periodic table, which arranged elements by atomic weight rather than atomic number.) There may be many numbers associated with each element, but the atomic number is always a positive whole number. If you know the name or symbol of the element, you can look up the atomic number on any periodic table.How you find the atomic number of an element depends on the information you’re given. Protons and neutrons each weigh about one atomic mass unit (amu), while the mass of an electron is only 0.000549 amu. Mass number does not include the mass of electrons because they are negligible compared to the mass of protons or neutrons.
ATOMIC NUMBER DEFINITION PLUS
Atomic number (A) is the number of protons plus neutrons, while mass number (Z) is the number of protons.
ATOMIC NUMBER DEFINITION FULL
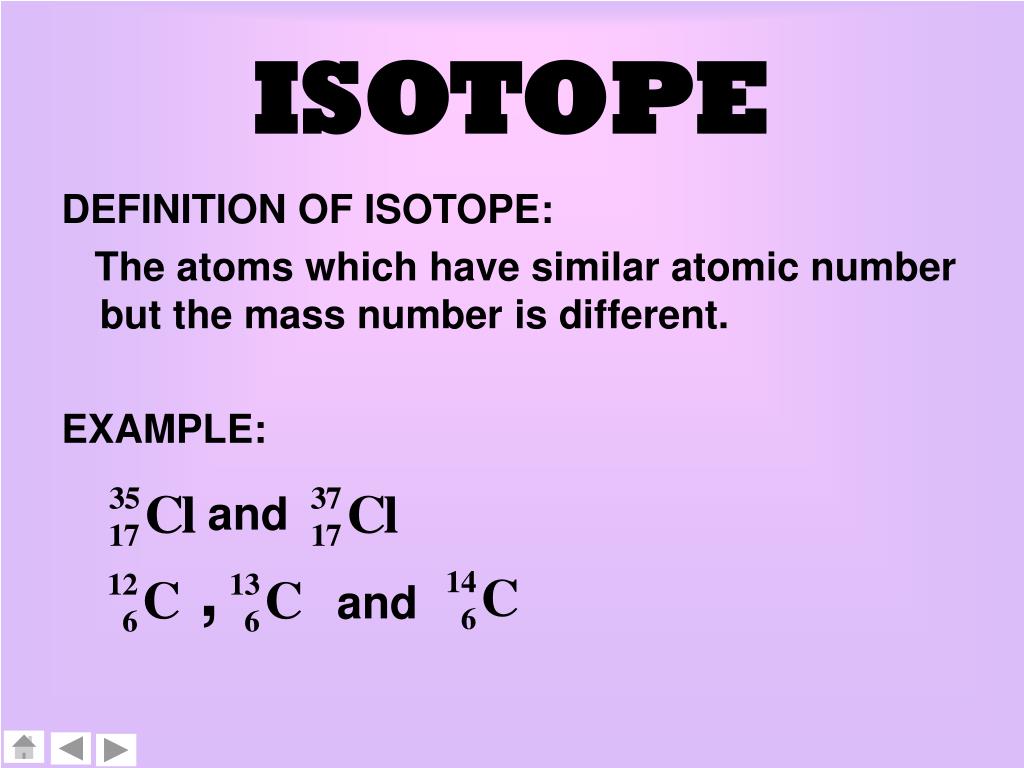
A full isotope symbol (A/Z format) includes both the atomic mass and atomic number (e.g., 14 6C, 12 6C). Mass number may be written after an element name or symbol (e.g., carbon-14) or as superscript above or to the left of an element symbol (e.g., 14C). Isotopes of an element have the same atomic number, but different mass numbers. The mass number identifies the isotope of an element. The symbol for mass number is A, which comes from the German word Atomgewcht (atomic weight). While the atomic number is the number of protons in an atom, the mass number is the sum of the number of protons and neutrons (the nucleons). When a new element is discovered, its atomic number will be the number of protons in its atomic nucleus.Ītomic numbers and symbols of all 118 elements Atomic Number vs Mass Number The periodic table currently has 118 atomic numbers. The number of neutrons and electrons doesn’t affect an atom’s identity, only its isotope and electrical charge, respectively. All atoms with atomic number 1 are hydrogen atoms all atoms with atomic number 118 are oganesson atoms. List of Atomic NumbersĪtomic numbers are always whole positive numbers. The valence electrons determine how readily an atom forms chemical bonds and the type of bonds it forms. This defines the atom’s electron configuration and the nature of its valence electron shell. The atomic number determines an element’s properties because its is the number of electrons in a neutral atom. Also, the periodic table is arranged in order of increasing atomic number. The atomic number is important because it identifies the element. In a neutral atom, the atomic number is equal to the number of electrons. The symbol Z comes from the German word zahl, which means numeral, or atomzahl, which means atomic number.īecause neutrons are neutral, the atomic number equals the electric charge of the atomic nucleus. It is denoted by the symbol Z and is the subscript in atomic notation. The atomic number is also called the proton number. The atomic number is the number of protons found in the nucleus of an atom, which uniquely identifies its element. This entry was posted on Octoby Anne Helmenstine (updated on August 26, 2021)


 0 kommentar(er)
0 kommentar(er)
